【Review of Fast-Charging Lithium-Ion Batteries】Mechanisms, Detection, and Mitigation of...

The rapid growth of the EV and energy storage industries is boosting demand for high-performance lithium batteries, driving the market for quality petroleum coke and synthetic graphite. The quality and particle size of calcined petroleum coke directly affect synthetic graphite performance, especially in anode production.
【Review of Fast-Charging Lithium-Ion Batteries】Mechanisms, Detection, and Mitigation of Lithium Plating on Graphite Anodes
This review article systematically examines one of the most critical challenges in fast charging of lithium-ion batteries: metallic lithium deposition (lithium plating) on the surface of graphite anodes. It not only provides an in-depth analysis of why lithium plating occurs, but also summarizes advanced techniques for detecting it and proposes a comprehensive set of solutions ranging from material modification to charging algorithm optimization. This is a timely and thorough industry review. One of the major pain points currently faced by the electric vehicle industry is slow charging. However, when charging current is aggressively increased, lithium ions cannot intercalate into the graphite anode in time and instead accumulate on the surface as metallic lithium, forming "dead lithium" or even dendrites, which can cause rapid capacity fade or, in severe cases, fires and explosions. The value of this article lies in its clear identification of the desolvation process as the primary bottleneck for fast charging. Rather than focusing on single-material improvements, it proposes a systematic, multi-dimensional anti-plating strategy involving electrolyte design, anode structural engineering, and charging protocol optimization, providing a clear technological roadmap for the development of next-generation extreme fast-charging (XFC) batteries.
Abstract
Lithium-ion batteries (LIBs) play a critical role in electric vehicles and energy storage systems, yet fast-charging technology still faces major challenges. Among them, lithium plating on graphite anodes is the core cause of performance degradation and safety risks. This review first analyzes the transport bottlenecks of lithium ions during fast charging that lead to lithium plating. It then summarizes the most advanced characterization and detection techniques for lithium plating. Subsequently, it comprehensively analyzes and evaluates various strategies for suppressing lithium plating, including novel electrolyte formulations, graphite anode modification, and optimized charging protocols. Finally, future directions for achieving safer and more efficient fast-charging batteries are discussed.
Introduction
Research Background:
With the expansion of the electric vehicle market, consumer demand for shorter charging times continues to increase (for example, the U.S. Department of Energy's target of charging to 80% state of charge within 15 minutes). However, under high-rate charging conditions, batteries experience severe internal polarization, which lowers the potential of the graphite anode and makes lithium plating more likely to occur. Lithium plating not only forms dead lithium that leads to irreversible capacity loss, but lithium dendrites may also pierce the separator, causing internal short circuits and thermal runaway. Therefore, lithium plating is a critical challenge that must be overcome in fast-charging technology.
Contributions of This Work:
Contribution 1: A detailed analysis of the five key steps of lithium-ion transport within the battery, clearly identifying desolvation and transport through the solid electrolyte interphase (SEI) as the key rate-limiting steps for fast charging.
Contribution 2: A systematic summary of lithium plating detection techniques, ranging from ex situ (post-mortem analysis) to in situ (real-time monitoring) methods, as well as electrochemical analysis approaches.
Contribution 3: A comprehensive evaluation of four major categories of lithium plating suppression strategies (electrolyte design, anode modification, charging protocol optimization, and other strategies), highlighting their respective advantages and limitations.
Experimental Design
Experimental Objects:
This review primarily focuses on lithium-ion batteries using graphite as the anode material, covering various cell formats from laboratory-scale coin cells to commercial pouch cells.
Test Conditions:
The review emphasizes extreme fast-charging (XFC) conditions, characterized by high charging current densities, as well as low-temperature environments (such as 0 °C and below), which are particularly prone to inducing lithium plating.
Research Methods
Core Concept / Overall Framework:
The article follows a closed-loop logic of mechanism analysis → detection techniques → mitigation strategies → future outlook. It first explains the causes of lithium plating from a microscopic kinetic perspective, then introduces methods to observe or detect lithium plating, and finally proposes strategies to prevent it.
Key Models / Theoretical Foundations:
1. Porous electrode theory and electrochemical polarization models: Used to explain the non-uniform lithium-ion concentration distribution across electrode thickness and why lithium plating tends to occur near the separator side.
2. Arrhenius equation: Used to analyze the effect of temperature on charge-transfer resistance and diffusion rates, revealing the kinetic limitations of low-temperature fast charging.
3. Nucleation and growth theory: Explains the deposition of metallic lithium on the graphite surface, as well as its nucleation and growth into dendrites.
Results and Discussion
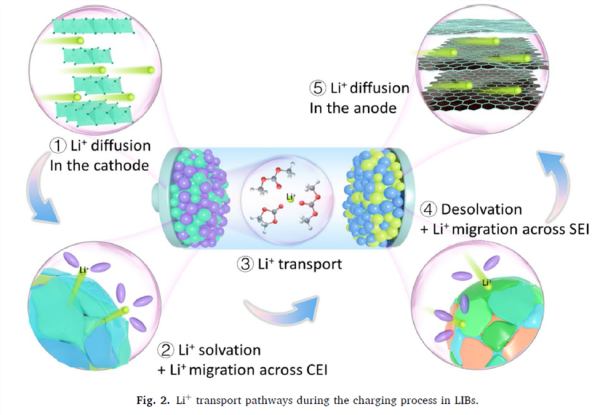
Figure 2 (Lithium-ion transport pathway):
Illustrates the five steps of lithium-ion transport from deintercalation at the cathode to intercalation into the anode. This figure shows that migration in the liquid phase and desolvation/transport through the SEI at the anode interface are the main rate-limiting steps.
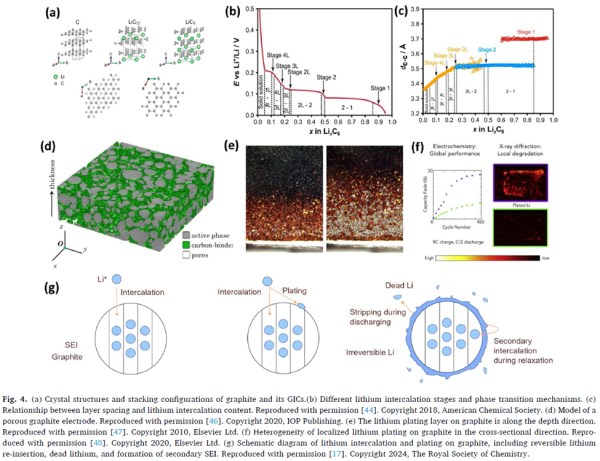
Figure 4 (Graphite intercalation and lithium plating mechanism):
Depicts the layered structure of graphite and different lithium intercalation stages, as well as local effects during lithium plating. This figure demonstrates that when the anode potential drops below 0 V (vs. Li/Li⁺) and surface lithium concentration becomes saturated, lithium ions are directly reduced to metallic lithium.
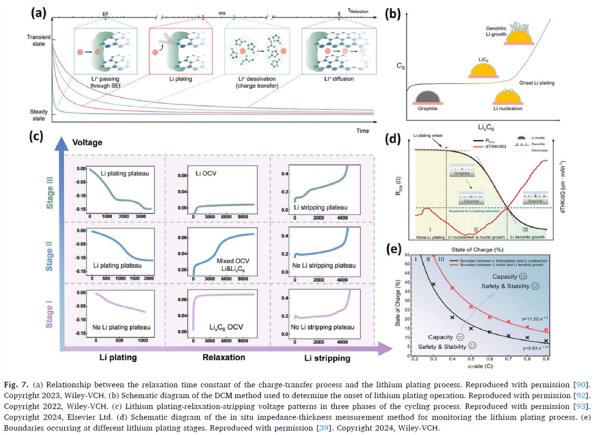
Figure 7 (Electrochemical detection methods):
Shows lithium plating detection through relaxation time constants, dynamic capacitance measurements, and differential voltage analysis. This figure illustrates that lithium plating can be identified without disassembling the battery, by analyzing subtle changes in voltage and impedance signals.
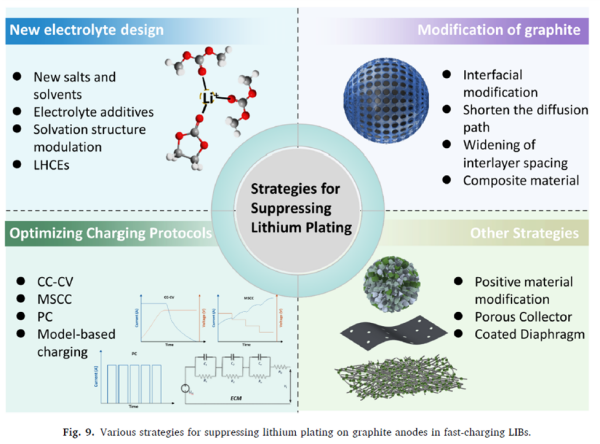
Figure 9 (Overview of mitigation strategies):
Summarizes new electrolytes, anode modification, charging protocol optimization, and other strategies. This figure visually presents the "toolbox" for addressing lithium plating, covering both materials and algorithms.
Main Findings
1. Kinetic bottleneck: The desolvation energy barrier of lithium ions in the electrolyte (the process by which lithium ions shed their solvent shell) is typically the largest obstacle limiting fast-charging rates, and its resistance is much higher than that of liquid-phase diffusion.
2. Evolution of lithium plating: Lithium plating can be divided into reversible active lithium (which can re-intercalate into graphite) and irreversible dead lithium and side-reaction products (which lead to permanent capacity loss).
3. Complementarity of detection techniques: A single detection method is often insufficient; accurate results require a combination of in situ physical characterization (such as optical microscopy and X-ray tomography) and electrochemical methods (such as three-electrode configurations and differential voltage analysis).
4. Multi-pronged approaches are more effective: Single material improvements (such as only modifying the anode) have limited effects. Combining high-conductivity electrolytes with intelligent charging strategies (such as pulse charging and temperature-adaptive charging) can significantly enhance fast-charging capability.
Authors' Interpretation:
The authors argue that although lithium plating thermodynamically occurs when the anode potential drops below 0 V, kinetic factors (such as local lithium-ion depletion and excessive interfacial resistance) dominate under practical fast-charging conditions. Reducing the desolvation energy barrier through localized high-concentration electrolytes (LHCEs), or adopting dual-layer graphite anode designs (a power-oriented top layer and an energy-oriented bottom layer), are currently highly promising solutions.
Conclusions
Core Conclusions:
Lithium plating on graphite anodes is the result of multi-physics coupling and is jointly influenced by temperature, charging rate, and material properties. Achieving truly safe fast charging requires moving beyond traditional trial-and-error approaches toward mechanism-based rational design. This includes accelerating ion transport by tuning solvation structures, mitigating polarization through structured anodes, and integrating intelligent battery management systems (BMS) for real-time monitoring of lithium plating boundaries.
Outlook
Areas for Improvement:
Among current detection techniques, ex situ methods damage battery structures and cannot reflect real operating conditions, while in situ techniques are often expensive and complex, making large-scale commercial implementation difficult.
Existing mitigation strategies are mostly localized optimizations and lack full-cell, system-level collaborative design encompassing the cathode, anode, electrolyte, and separator.
Future Directions:
1. Multi-scale collaborative optimization: Establish multi-physics models incorporating electrochemical, mechanical, and thermal effects to guide coordinated design of all battery components.
2. High-precision in situ characterization: Develop AI-assisted detection technologies that leverage multi-modal sensing to achieve real-time resolution of lithium plating nucleation and growth.
3. Intelligent charging systems: Develop low-cost embedded sensors and non-destructive detection algorithms integrated into BMS systems to enable simultaneous charging, monitoring, and adaptive control.
4. Integration with solid-state battery technologies: Although solid-state batteries can fundamentally eliminate leakage risks, their high interfacial resistance remains a challenge; future work should focus on improving fast-charging compatibility of solid electrolytes.
5. Sustainability: Develop bio-based solvents and other environmentally friendly materials, and address full life-cycle carbon emissions of fast-charging batteries.
Feel free to contact us anytime for more information about the Anode Material market. Our team is dedicated to providing you with in-depth insights and customized assistance based on your needs. Whether you have questions about product specifications, market trends, or pricing, we are here to help.
No related results found








0 Replies