【Anode Materials】Graphite-Based Anode Materials: The "Four Kings"

【Anode Materials】Graphite-Based Anode Materials: The "Four Kings"
Graphite, due to its high electronic conductivity, large lithium-ion diffusion coefficient, small volume change during lithium intercalation, high intercalation capacity (theoretical capacity of 372 mAh/g), and low intercalation potential, was the first commercially used anode material for lithium-ion batteries.
Since the development of lithium-ion batteries, various cathode material systems have been researched, but graphite-based anode material systems have remained in use. Graphite materials feature a low lithium intercalation potential and a layered structure conducive to lithium-ion intercalation and deintercalation. Current studies focus on graphitized carbon materials (natural flake graphite, graphitized mesocarbon microbeads, artificial graphite, etc.) and non-graphitized carbon materials (soft carbon, hard carbon, etc.). Against the backdrop of dual markets for power-type and consumer-type lithium batteries, artificial graphite and natural graphite anode materials have become mainstream in the market, consistently occupying over 90% of market share.
Graphite anodes account for about 12% to 21% of a lithium-ion battery by weight. Each pure electric vehicle contains approximately 50 kg of graphite, and each hybrid vehicle requires about 10 kg. Although the current supply exceeds demand, market demand will remain supported for a long time.
Mechanism of Lithium-Ion Batteries
During the charging process of a lithium-ion battery, lithium ions are smoothly extracted from the lattice of the cathode material (LiCoO3), gradually diffuse into the electrolyte, pass through the separator, and enter the graphite anode layers. Throughout this process, an equal number of electrons are released from the cathode and flow through the external circuit to the graphite anode to maintain charge balance, thereby forming a complete circuit. During discharge, lithium ions are deintercalated from the graphite layers, pass through the electrolyte, and return to embed into the LiCoO3 lattice, while electrons are transferred from the anode to the cathode via the external circuit, enabling the charge and discharge cycle.
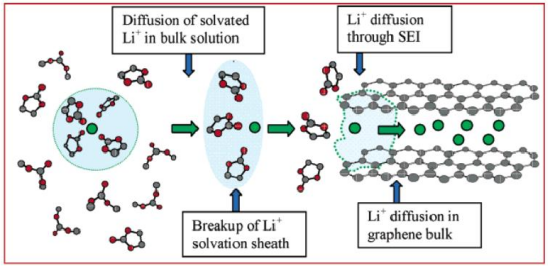
Charging Process of Graphite Anode
1. Artificial Graphite Anode Materials
Artificial graphite is obtained by graphitizing coke materials such as petroleum coke, pitch coke, metallurgical coke, mesocarbon microbeads, and needle coke at high temperatures. Among these, needle coke, a novel carbon material with excellent graphite microcrystal structure and needle-like texture orientation, is an ideal carbon source for producing lithium-ion battery anode materials. Its advantages include easy graphitization, high conductivity, relatively low cost, and low ash content. Additionally, it offers high lithium intercalation capacity, good lithium deintercalation reversibility, and meets the requirements for high voltage, large capacity, long cycle life, and high current density. Overall, artificial graphite outperforms natural graphite in initial Coulombic efficiency, rate performance, and cycle performance, making it the mainstream product in China's power-type lithium battery anode materials market in recent years.
However, using needle coke to produce artificial graphite anode materials has some drawbacks. These include irreversible reactions with electrolytes leading to decreased charge-discharge efficiency, reduced reversible capacity due to solvent co-intercalation, material volume expansion, and poor cycling performance. These challenges are bottlenecks for the further development of artificial graphite. Moreover, the preparation process requires extremely high temperatures (1,900–3,000°C) and a protective atmosphere, resulting in much higher costs than natural graphite. Therefore, cost reduction is an important research topic.
Research by Guo Mingcong and colleagues used raw coal-based needle coke and self-made high-performance coal tar pitch as a binder to prepare secondary particle artificial graphite anode materials with high energy density and rate performance. Their results showed that an 8% binder addition achieved optimal granulation, forming uniformly sized secondary particles with a tap density of 1.10 g/cm³, an initial charge capacity of 345.7 mAh/g at 0.1C, and an initial Coulombic efficiency of 95.6%, superior to materials with other binder amounts. The material also exhibited excellent high-rate charge-discharge capability during rate performance testing.
2. Natural Graphite Anode Materials
Natural graphite anode materials use flake graphite and microcrystalline graphite as raw materials. Due to the anisotropy and small interlayer spacing of flake graphite, its cycle and rate performance are poor when directly used as anode material. Therefore, it typically undergoes modification processes such as spheroidization, purification, and carbon coating. Spheroidizing natural flake graphite reduces its specific surface area, increases tap density, and improves lithium-ion diffusion in the anode material. Processing natural flake graphite into spherical graphite significantly enhances battery performance.
The spheroidization process for natural flake graphite mainly includes grinding and jet milling, both of which utilize high-carbon flake graphite as raw material. Mechanical forces such as collision, shearing, and friction in a spheronizer create uniform spherical graphite particles. After sufficient spheroidization, the product yield is typically only 40%-50%, with a large proportion of micropowder by-products, most of which are used for low-value-added products like lubricants and sealing materials.
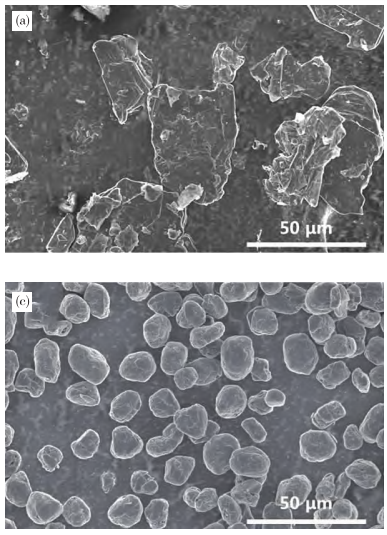
Scanning Electron Microscopy (SEM) Images of Flake Graphite and Spherical Graphite Anode Materials
Dong Huazhong and colleagues used graphite concentrates with approximately 95% fixed carbon content from Jixi, Inner Mongolia, and Luobei to prepare high-purity spherical graphite through spheroidization and chemical purification. Electrochemical testing showed that the initial charge-discharge efficiency of graphite anode materials from all three regions exceeded 90%, with an initial discharge capacity greater than 360 mAh/g, making them ideal raw materials for natural graphite anode production.
However, natural graphite anode materials, primarily derived from flake graphite, have several disadvantages:
1. Small specific surface area and low initial charge-discharge efficiency.
2. Anisotropy, hindering lithium-ion diffusion.
3. Cracks formed in the graphite layers due to lithium-ion intercalation and deintercalation, increasing diffusion resistance.
3. Amorphous Carbon
Amorphous carbon materials consist of amorphous carbon and graphite microcrystals, with numerous internal pores that serve as lithium storage sites during charge-discharge, increasing specific capacity. Thus, their theoretical specific capacity exceeds graphite's 372 mAh/g. Amorphous carbon includes soft carbon and hard carbon.
Soft Carbon: Soft carbon materials have abundant graphite-like microcrystalline regions arranged quasi-parallelly and can graphitize at high temperatures (>2,500°C). Typically derived from coke treated below 1,500°C, soft carbon features large interlayer spacing (>0.34 nm) and short-range order but exhibits low specific capacity (<300 mAh/g), low initial Coulombic efficiency (<80%), and low tap density. However, its advantages include excellent rate and long-cycle performance, offering significant commercial potential. Studies show that combining soft carbon with graphite can complement their strengths, enhancing battery rate and cycle performance.
Hard Carbon: Hard carbon materials cannot graphitize even above 3,000°C. Their structure includes curved graphite layers (pseudo-graphite regions) with larger interlayer spacing than graphite, forming two to six stacked layers. The structure also contains micropores that adsorb lithium ions during charge-discharge, although these pores lower initial Coulombic efficiency.
4. Graphene
Besides the materials mentioned above, other carbon-graphite-based anode materials for lithium-ion batteries include graphene and carbon fibers. Research by Zhang Tiange demonstrated that compared to traditional anode materials, two-dimensional Fe3O4/graphene composites exhibit excellent anti-polarization and conductivity, with superior electrochemical performance. Additionally, three-dimensional graphene network Fe3O4/graphene composites show slower capacity decay and better cycle stability and electrochemical performance, holding great application potential.
Outlook
In current lithium-ion battery material systems, next-generation anode materials such as silicon-carbon and lithium metal lack industrial maturity, are costly, and require compatible electrolyte and binder systems, limiting their ability to replace graphite-based anodes in the short term. Graphite-based anode materials will remain the market's dominant choice. However, significant changes may occur in the industrial structure. Under the "Carbon Peak, Carbon Neutrality" strategy and energy consumption "dual control" policies, natural graphite, with its lack of graphitization processes, low cost, and stable supply chain, is expected to see increased penetration in various applications.
Moreover, researchers worldwide have actively explored modification technologies to improve graphite anode performance, achieving notable results. Effective strategies for enhancing graphite anodes include reducing graphite particle size, doping, surface coating, co-intercalation of expanded graphite layers, and designing new electrolytes or additives to improve rate performance. Capacity enhancements can be achieved through doping and composite approaches, while cycle stability and safety improvements rely on surface coatings and electrolyte additives to stabilize graphite layer structures during charge-discharge and build robust SEI films.
Feel free to contact us anytime for more information about the artificial graphite anode market. Our team is dedicated to providing you with in-depth insights and customized assistance based on your needs. Whether you have questions about product specifications, market trends, or pricing, we are here to help.
No related results found








0 Replies